The pH level of sanitising products can help you pick the most suitable cleaning product for the job. Using the wrong cleaning chemicals in the commercial cleaning industry can cause thousands of pounds of damage and create a serious health risk.
Why is pH so important in cleaning?
PH levels play an important role in the effectiveness of cleaning products like surface cleaners, antiviral disinfectants, and industrial floor cleaners. However, understanding the pH level of your cleaning solutions is only half of the job. You must also know the pH level of the soil you are trying to clean so that you can use the opposite pH level.
We’ve written this article to give you a basic understanding of how pH level affects cleaning products, and how mastering this can benefit your commercial cleaning services.
What is the pH level of cleaning products?
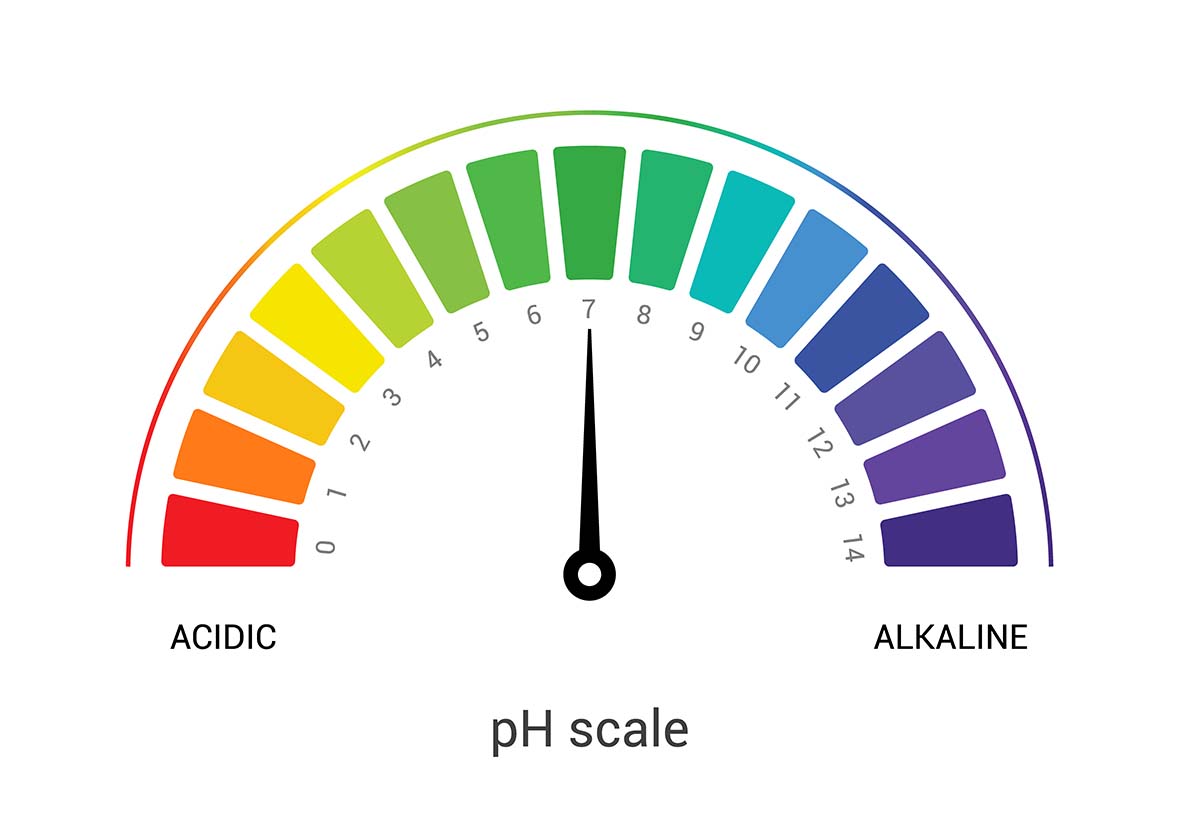
The pH scale gives liquids a rating of 0 to 14 depending on how acidic/basic the solution is.
- Substances with a rating of 0-6 are considered acidic e.g. lemon juice
- Substances with a rating of 8-14 are alkaline e.g. laundry detergent
- Pure water has a neutral pH of 7. Some other sanitising solutions, e.g. stone cleaners, also have a neutral pH level.
Why are most cleaning products basic?
Most cleaning chemicals are basic because alkaline solutions are the most effective at cleaning greases, oils, dirt stains, and other organics substances. On the other hand, acidic cleaning products are better at cleaning minerals like rust and calcium buildup.
How does pH affect cleaning products?
As a general rule, acidic cleaning chemicals are most effective at eliminating rust, calcium, and minerals. Whereas, an alkaline solution is better at removing grease, oil, and other organic materials.
Knowing the pH level of different pro clean products can be extremely useful in commercial cleaning. Below, are examples of common cleaning items and their disinfectant pH level:
Bleach (pH level 11 to 13)
The pH level of bleach is 11-13, making it one of the most alkaline cleaning products available. It is a powerful and effective antiviral disinfectant that removes bacteria, fungus, and viruses from surfaces. Bleach is highly corrosive and must be used with caution.
According to health and safety guidelines, you should always wear protective gloves when handling bleach and ensure that there is good ventilation.
Baking soda (pH level 8.5)
Baking soda has a pH level of around 8.5 which means it’s an alkaline solution and can be used for various cleaning tasks. A baking soda solution can be particularly effective at dissolving dirt, tackling odours, and removing grime.
Bleach should never be mixed with other cleaning chemicals as it can react and create toxic fumes.
Dish soap (pH level 7 to 8)
Mild dish soap is a neutral cleaning product and has the closest pH level to water.
This makes it safe to use for everyday cleaning, although it won’t be effective for commercial kitchen cleaning tasks or as a disinfectant for coronavirus, for example.
Toilet cleaner (pH level 1 to 3)
Toilet cleaner is a highly acidic cleaning product and has a pH disinfectant level of 1-3.
It can be used to remove rust, mineral deposits, and other non-organic materials. The hydrogen ions in acidic solutions react with the oxygen in metal oxides through the process called protonation.
As with bleach, toilet cleaners must be used with caution and proper ventilation is needed, along with suitable protective equipment e.g. gloves.
How do I choose the right pH?
There are several factors to consider when choosing the most suitable cleaning solutions based on pH level.
Soilage
Many commercial cleaning products are alkaline, as most soils and bacteria are eliminated most effectively with alkaline cleaning solutions. This includes cleaning items like floor cleaning products and surface cleaner.
Surface
You also need to consider the surface where you are applying the cleaning product. Sanitising solutions that are highly acidic or alkaline will typically damage fibres and dyes found in soft surfaces like carpets and furniture.
Tip: Always read the label on the back of commercial cleaning products to check what surfaces they can be used on.

Can I alter the pH level of cleaning solutions?
It is possible to alter the pH level of cleaning chemicals by adding solutions at the opposite end of the pH scale. For instance, a mildly acidic solution can be added to neutralise an alkaline substance.
Things to consider when altering pH levels
Altering the pH level of cleaning solutions will be counterproductive if you don’t know the pH level of the cleaning product you are using.
Changing the pH level of industrial cleaning supplies can also render the product ineffective, so it’s best to speak to professional cleaning suppliers if you’re unsure which cleaning solutions you should use based on pH level.
Safe handling of acid and alkaline cleaning products
- Always wear suitable PPE (personal protective equipment) like gloves and protective coveralls when using acidic and alkaline cleaners. These products are corrosive and must be handled with care.
- Make sure the cleaning product you are using is suitable for the surface you are treating e.g. an alkaline product like bleach is likely to stain fabrics so must be avoided.
- Read the label on the back on acidic and alkaline cleaning products carefully and follow the manufacturer’s guidelines.
- Avoid mixing acidic and alkaline products as they may lose their effect or release toxic chemicals.
Get in touch with Ultima for expert advice
At Ultima, we have over 20 years of experience tackling stains and tough cleaning tasks using different pH cleaning products.
If you need advice on which industrial cleaning supplies might be suitable for your next commercial cleaning job, then get in touch with our friendly, expert team today.
Looking for professional cleaning supplies?
You can also visit the Ultima online store to browse our collection of sanitising products and order UK cleaning supplies online. We also stock a wide range of professional sanitising equipment including fogging machines and personal protective equipment.





